Document Type : Original Article
Authors
1 Department of Biological Science Engineering, Faculty of Modern Science and Technologies, University of Tehran, Tehran, Iran
2 Research Assistant of the National Organization for the Development of Brilliant Talents, Alborz, Iran
3 Department of Chemistry, Khajeh Nasir Toosi University of Technology, Tehran, Iran
Abstract
The most important challenge in the fight against cancer is the direct delivery of drugs to cancer tissues and cells. In recent years, the use of nanoparticles as drug delivery systems has received attention. Drug delivery systems that respond to external factors such as temperature and pH are very useful in controlling drug release from nanocarriers. The aim of this research is to investigate the effect of pH and temperature simultaneously on the release of curcumin drug (CCM) from ZIF-8 nanoparticles. X-ray diffraction and scanning electron microscopy were used to identify nanoparticles. Biocompatibility of nanoparticles was evaluated using MTT method on MCF-7 cell line as breast cancer cell. The results showed that the preparation of smart nanoparticles ZIF-8 and ZIF-8 carrying CCM (ZIF-8@CCM) and sensitive to temperature and pH was successful. The diffusion rate of CCM was optimal at acidic pH and temperature of 50 °C. Also, ZIF-8@CCM nanoparticles prevented the growth of MCF-7 cancer cells. This study is completely new and innovative and shows that ZIF-8 nanoparticles sensitive to pH and temperature and carrying CCM are suitable candidates for the treatment of breast cancer cells.
Graphical Abstract
Keywords
Main Subjects
Introduction
Chemotherapy as a method to treat breast cancer has major challenges such as unpleasant effects, drug resistance and toxicity of healthy cells. Therefore, there is an essential need for anticancer treatment options with less drug resistance in addition to less toxicity [1,2]. The use of nanostructured systems in the targeted delivery of drugs to cancer tissues and cells is one of the most important treatment options to fight cancer. The targeted drug release is better than conventional drug treatment methods due to its ability to increase drug accumulation at the cancerous cells. However, current drug carriers have drawbacks that include short-term safety, biocompatibility, high production costs, and limited efficiency in drug delivery [2-4]. Until now, various organic and inorganic substances have been used for drug delivery systems. Organic materials usually have properties such as good biodegradability, low toxicity, and chemical modification capability, but they are not suitable for controlling the release rate of drugs. Instead inorganics have high photon output, optical stability, and favorable magnetic properties [5,6].
Metal-organic frameworks (MOFs) are special porous crystalline materials made of ordered bonds between different metal clusters (such as vertices) and multidimensional organic ligands. [3]. MOFs are widely used due to their special features such as large pore volume, high specific surface area, tunable pores, and high drug loading efficiency, and have become a useful material in the field of biomedicine [7].These nanoparticles release the drug in acidic environments, but one of their disadvantages is instability in physiological environments. MOFs are classified into two groups based on the ligands and metal ions used in their structure. One of the most widely used MOFs is zinc-based organometallic frameworks, which are composed of Zn2+ and imidazole or its derivatives [4].
Zeolite imidazole Frameworks-8 (ZIF-8) is one of the zinc-based MOFs that have attracted much attention for use in drug delivery systems [6]. ZIF-8 structures, as a subgroup of MOFs, are stable in aqueous solution, but when used as drug nanocarriers in acidic solution, the bond between zinc and imidazole ion is destroyed, causing drug to be released [2,3,8,9].
ZIF-8@drug
In the field of smart drug delivery to cancer cells, drug delivery systems based on pH-sensitive nanoparticles are suitable candidates, because the pH in cancerous tissue areas is around 5-6 and is more acidic than other tissues. These systems release the drug at the cancerous tissue more and faster than normal tissues [10-18]. Langer and Folkman were the first researchers to demonstrate the controlled release of macromolecules through polymers, and their action paved the way for the development of drug release systems in cancer therapy [10]. In 2015, ZIF-8 carrying the drug (ZIF-8@drug) doxorubicin was made [11]. Also in 2018, the drug release of 6-mercaptopurine from ZIF-8 nanoparticles was investigated and it was found that the drug release speed is higher at acidic environment [12]. In 2022, two drugs of 5-FU and LVCa were added simultaneously on ZIF-8 nanoparticles, which had additive efficacy [13]. In 2020, to increase the efficiency of lipophilic drugs in cancer treatment, the structure of ZIF-8 was used as a CCM drug carrier. In 2023, a nanocarrier based on ZIF-8 nanoparticles and gum arabic carrying CCM was prepared and used in cancer treatment [14]. Likewise, ZIF-8 was used as a hydrophobic D-α-tocopherol succinate carrier in drug release systems [15].
ZIF-8@CCM
The aim of this research is to design a targeted CCM delivery system based on ZIF-8 with anti-cancer application which has novelty. Initially, ZIF-8 nanoparticles carrying CCM were synthesized. The CCM was then incorporated into the structure of ZIF-8 nanoparticles, and then the final CCM delivery system was evaluated with pH change and temperature stimuli in terms of controlled release. Finally, the possibility of using CCM delivery system was investigated in the treatment of breast cancer.
Experimental
Material and instrumental
Materials of zinc nitrate Zn (NO3)2.6H2O, 2- methylimidazole, methanol, and curcumin (CCM). Field Emission Scanning Electron Microscope (FESEM), VEGA3-MIRA II-MIRAIII model (TESCAN) was used to examine the morphology of ZIF-8 and ZIF-8@CCM nanoparticles. X-Ray Diffraction (XRD) analysis with PW1730 (PHILIPS) model was used to investigate the microscopic structure of crystals and identify ZIF-8 and ZIF-8@CCM nanoparticles. XRD was created with a wavelength of 1.54 Angstroms and a CuKα radiation source, and the 2Theta diffraction angle was changed in the range of 40 to 50 degrees. Ultraviolet- Visible (U-Vis) spectroscopy with Biomate5 model (Thermo) was used to check CCM release at 420 nm. Centrifuge with model WISDWOF (HETTICH) and Eliza reader modeELx808 (Biotek) was used.
Preparation method of ZIF-8@CCM
The value of 0.32 g of Zn (NO3)2.6H2O was added to 10 ml of methanol and 0.6 g of 2-methylimidazole was added to 10 mL of methanol and both solutions were vigorously stirred for one hr at room temperature. After that, Zn (NO3)2.6H2O solution was added drop by drop to the 2-methylimidazole solution and stirred for one hour. The milky mixture remained at room temperature for 12 hr (Figure 1a), and then the mixture was centrifuged for 20 min at 8000 rpm to separate the white nanoparticles. The white nanoparticles were washed three times with methanol. After that, the ZIF-8 nanoparticles were placed in the oven at 120 °C for 12 hr to remove excess solvent and activate it. To prepare ZIF-8 nanoparticles carrying CCM (ZIF-8@CCM), 10 mg of CCM was dissolved in deionized water and added to methanol solution of 2-methylimidazole (Figure 1b). Thereafter, all the previous steps were repeated to prepare nanoparticles containing CCM (ZIF-8@CCM).
 Figure 1. (a) Mixture of Zn (NO3)2.6H2O and 2-methylimidazole and (b) Adding CCM to zinc nitrate and 2-methylimidazole solution.
Figure 1. (a) Mixture of Zn (NO3)2.6H2O and 2-methylimidazole and (b) Adding CCM to zinc nitrate and 2-methylimidazole solution.
CCM release test
After the end of the loading process, to separate unloaded CCM from the ZIF-8@CCM nanoparticles, the solution was centrifuged for 20 min at 8000 rpm. The remained CCM was measured using a spectrophotometer at 420 nm [1] and according to the CCM calibration curve in Figure 2.
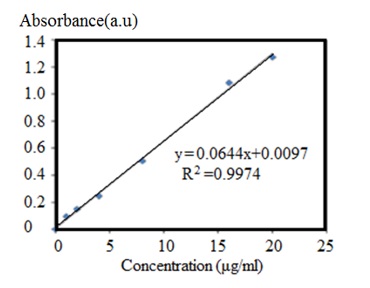
Figure 2. CCM calibration diagram
Assessment of cytotoxicity by MTT method
The MCF-7 cells obtained from the cell bank of Pasteur Institute of Iran were used to investigate cytotoxicity. After thawing, cells were transferred to a flask containing Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS). The flask was placed in an incubator with a temperature of 37 °C, 5% carbon dioxide concentration and 90% humidity. The culture medium was changed every 4 days. The samples were first sterilized with alcohol to check their toxicity and effect on cell proliferation and growth. The extraction process was carried out according to the ISO-5-10993 standard. One milliliter of culture medium was added to all sterile samples for 0.1 g, and then after 3 and 7 days, the culture medium was taken out and added to the cells. A certain amount of DMEM culture was included as a control sample.
One of the most suitable indirect methods available to find cell proliferation is the dimethyl thiazyl diphenyl tetrazolium bromide (MTT, Sigma, USA) test. This method is based on the conversion of yellow tetrazolium powder into purple insoluble crystals and black formazan crystals. This happens only in living cells using an enzyme called succinate dehydrogenase.
In this study, first, 1*104 cells along with 100 µl of culture medium were transferred into each well of a 96-well cell culture plate. Then they were placed in an incubator at 37 °C for 24 hr. This was done to check the rate of cell proliferation. Cells stick to the bottom of the page. After ensuring the adhesion of the cells, the culture medium on the cells was removed as possible, and 90 µl of the extract of each sample with 10 µl of FBS were poured to culture wells. After that, the cells were placed in the vicinity of the wells for another 24 hr.
Finally, 100 μl of MTT with a concentration of 0.5 mg/mL was poured into each well and incubated for 4 hr. After 4 hr, the solution was removed from the cells and isopropanol was added until the purple crystals were dissolved. The concentration of the substance dissolved in isopropanol was calculated at a wavelength of 570 nm with an Elaiser device. A well with fewer cells showed a lower optical density (OD), which can be inferred that the number of living cells is reduced and growth is prevented.
Results and Discussion
FESEM of ZIF-8 and ZIF-8@CCM nanoparticles
FESEM was used to examine the morphology and size of ZIF-8 and ZIF-8@CCM nanoparticles. The size was exactly measured using Image J software. As demonstrated in the Figure 3, the CCM presence in the structure of ZIF-8 increased the size of nanoparticles from about 100-150 nm (Figure 3a) to about 300-320 nm (Figure 3b). Also, the dispersion of nanoparticles indicates the synthesis of homogeneous and uniform nanoparticles. In general, these nanoparticles have desirable and required properties for CCM delivery purposes.
XRD results of ZIF-8 and ZIF-8@CCM nanoparticles
Figure 4 shows the XRD of ZIF-8 and ZIF-8@CCM nanoparticles, which corresponds to the reported pattern of the ZIF-8 nanoparticles [1,3,14]. The addition of CCM to ZIF-8 nanoparticles increases the distance between the crystal plates of nanoparticles and as a result, the diffraction peaks in the highest peak (011) move to the left and at a lower angle. In general, due to the introduction of CCM into ZIF-8 nanoparticles, there was no significant difference in the structure and the atomic order has been maintained. The peaks are observed at 2θ = 7.29, 10.34, 12.69, 14.79, 16.42, and 18.32 degrees, which correspond to plates (110), (200), (211), (220), (310), and 222, respectively. These observations indicates the high crystallinity of ZIF-8 prepared correspond to JCPDS is 00-062-1030 index number.
 Figure 3. FESEM of (a) ZIF-8 and (b) ZIF-8@CCM nanoparticles.
Figure 3. FESEM of (a) ZIF-8 and (b) ZIF-8@CCM nanoparticles.

Figure 4. XRD of ZIF-8 and ZIF-8@CCM nanoparticles.
Investigation of CCM release test
In this section, an attempt has been made to answer the question whether the final CCM delivery system shows controlled self-release by changing pH and temperature. Kinetics of CCM release from ZIF-8@CCM nanoparticles were investigated at neutral conditions (pH = 7.4) with compared to acidic condition (pH=5) which is suitable for cancerous microenvironment, as depicted in Figures 5 and 6. Also, two temperatures of 37 °C and 50 °C were considered simultaneously to investigate the sensitivity of CCM release to temperature. At higher temperature (50 °C), the amount of CCM release from ZIF-8@CCM nanoparticles increased, as depicted in Figure 5. As shown in Figure 6, the release rate of CCM from ZIF-8 framework is faster under acidic conditions than under physiological conditions. The reason can be the breaking of the bond between Zn2+ ions and methyl-imidazole in acidic solution. These results are good reason for the use of ZIF-8 nanoparticles in the treatment of cancerous tissue that has a higher temperature and acidic pH. In general, by changing the pH and temperature stimuli, the CCM delivery system would have a controlled release condition.

Figure 5. CCM release diagram from ZIF-8@CCM nanoparticles at pH=7.4 and 37 and 50 °C.
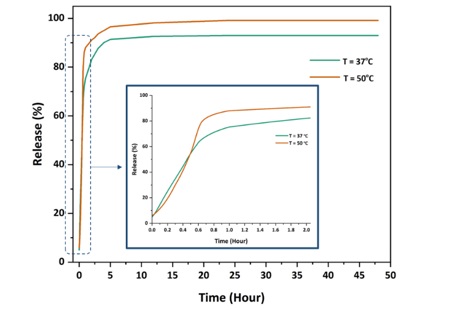
Figure 6. CCM release diagram from ZIF-8@CCM nanoparticles at pH=5 and 37 and 50 °C.
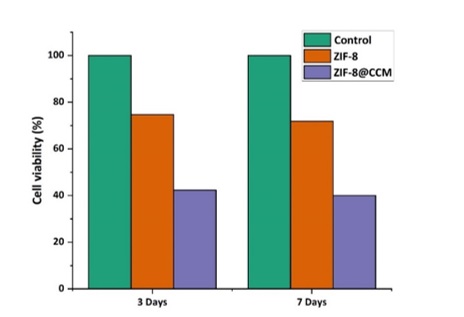
Figure 7. MTT results of ZIF-8 and ZIF-8@CCM nanoparticles.
MTT test review
The results of the MTT cytotoxicity test at three days and seven days are presented in the Figure 7. The number of live MCF-7 cells was measured in two samples of ZIF-8 and ZIF-8@CCM nanoparticles. As shown in the diagram, the number of viable cancer cells in cases where ZIF-8 nanoparticles containing CCM is less than the control (a certain amount of culture of DMEM) and ZIF-8 nanoparticles were used alone. This can be the reason for the anticancer properties of CCM and its effectiveness.
Conclusion
In this research, ZIF-8 nanoparticles carrying the CCM (ZIF-8@CCM) were used in the treatment of breast cancer. The simultaneous effect of temperature and pH stimuli was investigated in controlling CCM release from ZIF-8@CCM. The dimensions of ZIF-8 nanoparticles were about 100-150 nm and ZIF-8@CCM nanoparticles were about 300-320 nm. The results of FESEM and XRD showed the successful synthesis of ZIF-8 nanoparticles and were similar to the articles. The XRD results similar to the literature confirmed the presence of CCM in the structure of ZIF-8 nanoparticles. The results of the CCM release test showed that at a temperature of 50 °C and acidic pH, there is the highest CCM release rate from nanoparticles, which can have a controlled CCM release in cancerous tissue. The results of the MTT test confirmed the effectiveness of CCM-carrying nanoparticles in killing MCF-7 breast cancer cells. In general, it can be said that ZIF-8 nanoparticles carrying CCM, in addition to targeted drug delivery to cancer tissue, can reduce the side effects of anticancer drugs and cause minimal damage to surrounding healthy tissues. Although there are still challenges and limitations in the use of nanoparticles in treatment, we hope that in the near future they can be used in medical science and therapeutic purposes .
Acknowledgment
We are grateful to all those who sincerely helped us in conducting this research. Those who became our stairs to climb more easily and did not expect us, not even a thank you. We are grateful to all our fathers and mothers and all our predecessors who blessed us with their patience.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Orcid
Pegah Shams : 0009-0008-1946-1161
Hedyeh Panahi : 0009-0006-3704-1940
Masoume Tahan : 0009-0007-5242-105X
Fatemeh Jahansooz : 0000-0002-9582-4920
Ashraf Heidaripour : 0000-0003-0223-5383


