Document Type : Original Article
Authors
Department of Chemistry, Faculty of Science, Golestan University, Gorgan, Iran
Abstract
New modified chitosan Schiff base (EP-CS-SB) composite was prepared from the reaction of chitosan, epichlorohydrin and trans-cinnamaldehyde (molar ratio 1:1:1) and characterized by Fourier-transform infrared spectroscopy (FT-IR), ultraviolet–visible spectroscopy (UV-Vis), thermogravimetry and differential thermal analysis (TG-DTA), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and scanning electron microscope (SEM) analysis. It was applied as a new adsorbent for removal of methyl green (MG) dye. The effects of different significant parameters, such as pH solution (2-10), adsorbent dosage (0.005, 0.01 and 0.02 g) and contact time (0 – 120 min) were explored. Results show that the adsorption process was depended on pH solution. The highest adsorption capacity of EP-CS-SB was obtained 98.47% at pH =8, 120 min, and 0.02 g of EP-CS-SB. EP-CS-SB was found to be a suitable candidate for removal of cationic dye.
Graphical Abstract
Keywords
Introduction
In recent years, water pollution by various compound such as organic dyes and heavy metal ions, is a great threat for human health and environment [1‒6]. Organic dyes are cheap, more stable and also used to in various industries [1‒4] and can damages the balance of the ecosystem [2]. Until now, several techniques and various materials used to degradation and removal of organic dyes from aqueous solution [7‒22]. From the various techniques, the adsorption route is one of the most promising technique due to simple, highly efficient and low-cost [1‒4, 9‒14, 19]. Yin et al. [19] synthesized and used the magnetic CoFe2O4/rGO nanocomposites for methyl orange and methyl green dyes. Sahoo et al. [18] synthesized SrFe12O19 using sonochemical route and used to removal of malachite green as cationic dye.
Chitosan, is eco-friendly and nontoxic [2], and is containing many amine and hydroxyl groups used as a good adsorbent for organic dyes removal [2‒5, 11‒14]. H2SO4 crosslinked magnetic chitosan nanocomposite beads prepare by Rahmi et al. [2] and used for removal of methylene blue. Results demonstrated that the maximum adsorption capacity is 20.408 mg/g. The maximum adsorption capacity of 189.44 mg/g for RB5 has been reported using nano-ZnO/chitosan composite beads [4]. Chitosan easily modified by terephthaldehyde [23], glutaric acid [24] and epichlorohydrin [25], to improve its properties.
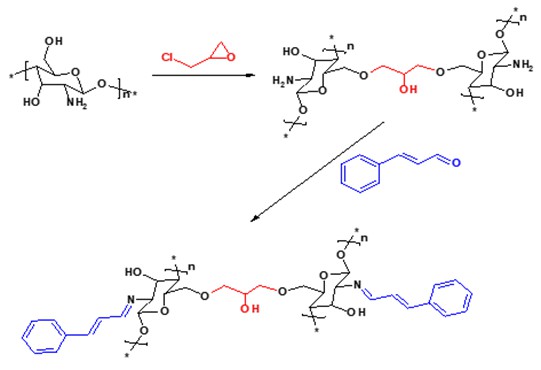
Continuing the previous work [26‒28], new modified chitosan Schiff base (EP-CS-SB) (Scheme 1) was prepared and characterized. In addition, removal of methyl green from aqueous solution has been evaluated.
Experimental
Materials and methods
All compounds were purchased from the Sigma-Aldrich Company. FT-IR spectrum was recorded using Perkin-Elmer FT-IR spectrophotometer. TG/DTA and DSC curves were recorded using Perkin-Elmer TG-DTA analyzer and DSC analyzer Model 60A (Shimadzu), respectively. XRD pattern was determined by Bruker AXS-D8 X-ray diffractometer. SEM image was recorded on the TESCAN Vega Model scanning electron microscope. UV-Vis spectra were carried out with UV-Visible spectrophotometer (Perkin-Elmer).
Synthesis of EP-CS-SB
1 g of chitosan was suspended in 25 mL of ethanol-glacial acetic acid (95:5 v/v) for 10 min. Epichlorohydrin (1 mL in 10 mL ethanol) was added and the mixture was stirred for 6 h. Then, 2 mL trans-cinnamaldehyde was added dropwise and refluxed for about 12 h. The pale-yellow solids were filtered, washed and dried.
 Scheme 1. Preparation of modified chitosan Schiff base (EP-CS-SB)
Scheme 1. Preparation of modified chitosan Schiff base (EP-CS-SB)
Adsorption experiments
All adsorption experiments were carried out according to previous paper [26‒28]. To 20 mL of methyl green solution with initial concentration of 40 mg/L, various dosages of EP-CS-SB (0.005, .01 and .02 g) were added. The solution was shacked and at various contact time, EP-CS-SB was centrifuged and UV-Vis spectrum was recorded. The removal percentage (%) was calculated using the Equation 1.
R% = {(Ci-Ct) × 100} / Ci (1)
Where Ci and Ct (mg/L) are the initial and final concentration of methyl green
Results and Discussion
Characterization of EP-CS-SB
In the FT-IR spectrum of EP-CS-SB (Figure 1), there are several peaks. The weak and broad peak at 2861 cm-1 is assigned to the C-H stretching [11, 26‒28]. The sharp peaks at about 1632 and 1060 cm-1, are assigned to the C=N iminic stretching [11, 26‒28] and C-O-C stretching in the pyranose ring [3, 4].
TG-DTA curves (Figure 2) illustrates that EP-CS-SB is stable until 75 °C. In the 1st stage (from 75 to 150 °C), it shows<5% of mass losses, corresponds to the ethanol molecules adsorbed on EC-CS-SB [29]. This stage is endothermic. In the temperature ranges of 150-400 °C, EP-CS-SB shows mass losses of ≈50%, attributed to the exothermic degradation of polymeric chain. Finally, mass losses of ≈17% happened from 400 to 825 °C, due to decomposition of residual crosslinked of EP-CS-SB [29].
 Figure 1. FT-IR spectrum of EP-CS-SB
Figure 1. FT-IR spectrum of EP-CS-SB
In the DSC thermogram (Figure 3), there are endothermic peak (84.5 °C) and exothermic peak (277.5 °C), assigned to the evaporation of ethanol molecules [26, 30], decomposition of polymeric chain [26, 30], respectively.
In the XRD pattern of EP-CS-SB (Figure 4), there are two peaks at about 11.29° and 20.48°. The peak at 11.29° confirm the modification of chitosan. The peak at 20.48 confirmed the presence of chitosan [4, 14]. SEM image of EP-CS-SB depicted in Figure 5. It can be seen that the surface of EP-CS-SB is coarse and porous.
 Figure 2. TG-DTA curves of EP-CS-SB
Figure 2. TG-DTA curves of EP-CS-SB
 Figure 3. DSC thermogram of EP-CS-SB
Figure 3. DSC thermogram of EP-CS-SB
 Figure 4. XRD pattern of EP-CS-SB
Figure 4. XRD pattern of EP-CS-SB
 Figure 5. SEM image of EP-CS-SB
Figure 5. SEM image of EP-CS-SB
Adsorption studies
The pH of solution is an important parameter to removal of methyl green dye [19, 26, 27, 31‒39]. The maximum methyl green removal was observed in the pH of 6-8 like those reported for MG using CoFe2O4/rGO [19], activated carbon [34], activated bentonite [33], holloysite nanotube [36] and modified chitosan Schiff base [26]. The effect of initial pH (from 2 to 10) on the removal percentage of MG shown in Figure 6. In the pH of 8, about 98.47% of methyl green has been removed, due to the electrostatic attraction of partial δ- surface charge of EP-CS-SB and the positive charge of methyl green [26].
The effect EP-CS-SB dosages on the percentage removal of methyl green at different contact times are revealed in Figure 7. The methyl green removal is very fast at the beginning (1 min) of the reaction, due to the high active sites of EP-CS-SB. Also, by increasing of the EP-CS-SB dosage and the contact time, the percentage removal of MG was increased [26, 27, 30, 31].
The maximum of methyl green removal percentage (%) on the surfaces of EP-CS-SB, was compared with those reported in the similar papers using different adsorbent and shown in Table 1.
 Figure 6. Effect of initial pH on removal percentage of MG
Figure 6. Effect of initial pH on removal percentage of MG
 Figure 7. Effect of EP-CS-SB dosage and contact time on the percentage removal of MG
Figure 7. Effect of EP-CS-SB dosage and contact time on the percentage removal of MG
Table 1. Comparison of removal percentage (%) of MG using various adsorbents

Conclusions
New modified chitosan Schiff base (EP-CS-SB) was synthesized and characterized using various techniques. The FT-IR and XRD results confirmed the preparation of EP-CS-SB. The title compound EP-CS-SB was applied as an effective adsorbent for the removal of methyl green. The optimum condition for the methyl green removal were pH solution of 8, 0.02 g adsorbent dosage, initial concentration of MG is 40 ppm and 120 min contact time. At this condition, the maximum removed percentage of methyl green achieved up to 98.47%. Therefore, EP-CS-SB can be used as an environmentally friendly good adsorbent.
Acknowledgments
The authors acknowledge the Golestan University for supporting this work.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Orcid
Aliakbar Dehno Khalaji : 0000-0002-8362-5158