Document Type : Original Article
Author
Department of Chemistry, Ardabil Branch, Islamic Azad University, Ardabil, Iran
Abstract
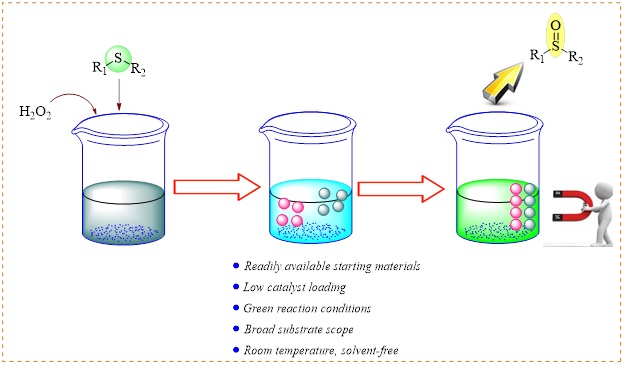
In the present study, Zn–DABCO@Fe3O4 with a high surface area and readily was synthesized. The morphology, particle size distribution, and phase analysis of the Zn–DABCO@Fe3O4 nanopowders were characterized using the scanning electron microscope (SEM), transmission electron microscope (TEM), vibrating sample magnetometer (VSM) and X-ray diffraction (XRD) analysis. The prepared Zn–DABCO@Fe3O4 nanoparticle was utilized as efficient catalyst for selective oxidation of sulfides to sulfoxides using 30% H2O2 as oxidant. The products were achieved with good to excellent yields at room temperature with no over-oxidation of sulfoxides and disulfides to unexpected by-products. This catalyst can be magnetically recovered by applying an external magnet and reused for eight continuous cycles in both oxidation reactions without a notable loss in its catalytic activity. Furthermore, the oxidation of various sulfides was highly chemoselective, but O,O-acetals remained intact under the described reaction conditions.
Graphical Abstract
Keywords
Introduction
Nano magnetic based heterogeneous catalytic systems have a high surface-to-volume ratio, which guarantees the catalytic system's high activity. Also, these catalysts can be easily separated from the reaction media by applying a simple external magnet. These features make nano magnetic based heterogeneous catalytic systems as a great option for both industrial and academic chemists [1⎼7]. The magnetic nanoparticles can be utilized in various organic reactions such as synthesis of a-amino nitriles [8], nucleophilic substitution reactions of benzyl halides [9], Suzuki coupling reactions [10], esterifications [11], knoevenagel reaction [12], hydrogenation of alkynes [13], epoxidation of alkenes [14], CO2 cycloaddition reactions [15], and three-component condensations [16]. In addition, MNPs were emerged widely for various areas applications including, waste-water treatment [17], sonochemical approach [18], electrochemical storage of hydrogen [19], magnetic resonance imaging [20], drug delivery [21], biology and medical applications [22], and magnetic fluids [23].
The selective oxidation of sulfides into the corresponding sulfoxides has taken up an outstanding part in modern synthetic organic chemistry [24]. Organic sulfoxides play an essential role in synthesizing chemically useful and biologically active compounds, including drugs, flavours, germicides, and catabolism regulators [25]. In recent years, some procedures have been reported for the oxidation of sulfides to sulfoxides such as H2O2/Mn(III) [26], amberlyst 15/H2O2 [27], vanadium(IV) complexes of dibromo- and diiodo-functionalized chiral Schiff bases [28], NBS/b-cyclodextrine [29], silica-based tungstate/H2O2 [30], Organic–Inorganic Polyoxometalate-Based Frameworks [31], MNPs-supported acidic catalysts [32], SBA-15 Based Tungstate/H2O2 [33], Cu Salen-Fe3O4/H2O2 [34], pre-formed manganese complex/H2O2 [35], γ-Fe2O3@HAp-Ag NPs [36], molybdate-based catalyst/ H2O2 [37], and metalloporphyrins immobilized into montmorillonite/H2O2 [38]. Unfortunately, some methods based on sulfides' oxidation require hazardous and toxic reagents or include over-oxidation of sulfides and thiols to undesired by-products. Therefore, further progress in eco-friendly selective oxidation of sulfides to sulfoxides using less poisonous catalysts, oxidants, and solvents is still needed.
In this work, Zn–DABCO@Fe3O4 was synthesized (Scheme 1). The prepared Zn–DABCO@Fe3O4 nanoparticles were fully characterized using various physicochemical methods and successfully used for selective oxidation of sulfides to sulfoxides at room temperature (Scheme 2).
 Scheme 1. The synthesis of Zn–DABCO@Fe3O4 MNPs
Scheme 1. The synthesis of Zn–DABCO@Fe3O4 MNPs
 Scheme 2. Zn–DABCO@Fe3O4 MNPs catalyzed oxidation of sulfides to sulfoxides at room temperature
Scheme 2. Zn–DABCO@Fe3O4 MNPs catalyzed oxidation of sulfides to sulfoxides at room temperature
Experimental
Materials and methods
All reagents and used materials were purchased from the Sigma-Aldrich and Merck, and were utilized any additional purification. Products were separated and purified by different chromatographic techniques and were identified by the comparison of their IR, NMR, and melting point with those reported for the authentic samples. All the melting points were recorded by open capillary method and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance (250 MHz) spectrometer using CDCl3 as the solvent and TMS as an internal standard. Thin-layer chromatography (TLC) was performed on pre-coated aluminium plates (silica gel 60 F254, Merck). IR spectra of the compounds were obtained on a Perkin Elmer spectrometer version 10.03.06 using a KBr disk. The phases present in the magnetic materials were analyzed using a powder XRD, Philips (Holland), model X0 Pert with X´ Pert with CuKα1 radiation (λ = 1.5401 Å), and the X-ray generator was operated at 40 kV and 30 mA. Diffraction patterns were collected from 2h = 20º–80º. The mean size and the surface morphology of the nanoparticles were characterized by FT-IR, TEM, SEM, VSM, and XRD.
Synthesis of Zn–DABCO@Fe3O4
DABCO@Fe3O4 was prepared via the condensation of Fe3O4 MNPs with n-propyl-4-aza-1-azoniabicyclo[2.2.2]octane according to the literature procedure [39]. A mixture of DABCO@Fe3O4 and Zn(OAc)2 in dry acetone (20 mL) was stirred at room temperature for 24 h. The solid product was filtered by suction, washed with acetone, distilled water and acetone successively and dried under vacuum at 60 °C for 4 h to give complex of Zn–DABCO@Fe3O4 (2.03 g).
General procedure for oxidation of sulfides to sulfoxide
In a typical procedure, 15 mg of the prepared Zn–DABCO@ Fe3O4 nanocatalyst, 1 mmol of sulfide and 0.5 mL of 30% H2O2 were mixed at room temperature for the time specified in Table 3. This mixture was magnetically stirred at room temperature and the reaction progress was checked by thin-layer chromatography (TLC). After completion of the reaction, the catalyst was magnetically collected and was washed with CH2Cl2 and dried. Lastly, to achieve the pure product, it was extracted with CH2Cl2 (3 × 10 mL) and purified on a silica-gel column and products were obtained in 82–99% yield.
Results and Discussion
The successful preparation of novel acidic nanomagnetic catalyst was investigated and confirmed by applying several skills such as X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscope (SEM) and vibrating sample magnetometer (VSM) analysis. In the following, the obtained data were investigated in detail.
Characterization of Zn–DABCO@Fe3O4 MNPs
The structural properties of synthesized Fe3O4 MNPs (A), DABCO supported with Fe3O4 MNPs (B), and Zn–DABCO@Fe3O4 (C) were analyzed by XRD (Figure 1). As seen in Figure 1, the XRD patterns of the synthesized Zn–DABCO@Fe3O4 display several relatively strong reflection peaks in the 2 h region of 20–80o, which is quite similar to those of Fe3O4 MNPs, DABCO supported with Fe3O4 MNPs reported by other group. The XRD patterns of the particles demonstrated characteristic peaks at (220), (311), (400), (422), (511), and (440), which match well with the database of magnetite (JCPDS Card: 19-629) file.
The nanostructure of the Zn-DABCO@Fe3O4 MNPs was confirmed by transmission electron microscopy (TEM) (Figure 2c). The TEM micrograph clearly proved that the particles were in nanosize. The average particle size of the Fe3O4 MNPs was found to be 25-30 nm (Figure 2a). After being coated with a DABCO, the typical core–shell structure of the Fe3O4-DABCO can be observed, and the average size increases to about 45-50 nm (Figure 2b). Zn-DABCO@Fe3O4 NPs (Figure 2c) consist of relatively small, nearly spherical particles, with an average size of 30 nm, much smaller than the sizes obtained from the XRD measurements.
The scanning electron microscope (SEM) micrographs of the Fe3O4 MNP (Figure 3a), DABCO supported with Fe3O4 MNPs (Figure 3b), and Zn-DABCO@Fe3O4 NPs (Figure 3c) revealed that the particles of the catalyst were observed in nanosize.
In another investigation, magnetic measurements of Zn-DABCO@Fe3O4 MNPs were performed at room temperature using a vibrating sample magnetometer (VSM). The magnetization curve in Figure 4 gives a saturation magnetization value of 64 emu/g (Figure 4).
 Figure 1. XRD patterns of a) Fe3O4 MNP, b) DABCO supported with Fe3O4 MNPs, and c) Zn–DABCO@Fe3O4
Figure 1. XRD patterns of a) Fe3O4 MNP, b) DABCO supported with Fe3O4 MNPs, and c) Zn–DABCO@Fe3O4
 Figure 2. TEM images of a) Fe3O4 MNPs, and b) DABCO@Fe3O4 and c) Zn–DABCO@Fe3O4
Figure 2. TEM images of a) Fe3O4 MNPs, and b) DABCO@Fe3O4 and c) Zn–DABCO@Fe3O4
 Figure 3. The SEM micrographs of a) Fe3O4 MNPs, b) DABCO@Fe3O4, and c) Zn–DABCO@Fe3O4
Figure 3. The SEM micrographs of a) Fe3O4 MNPs, b) DABCO@Fe3O4, and c) Zn–DABCO@Fe3O4
 Figure 4. Magnetization curve of Zn-DABCO@Fe3O4 NPs
Figure 4. Magnetization curve of Zn-DABCO@Fe3O4 NPs
Scope of reaction
After characterization of the synthesized Zn-DABCO@Fe3O4 NPs, its catalytic activity was evaluated in the oxidation of sulfides to provide sulfoxides to find the best reaction conditions, the effect of the different experimental parameters (e.g. effect of solvent and catalyst) on the reaction efficiency were studied. Evidently, in the lack of the catalyst, the reaction could not progress by 120 min (Table 1, entry 1). The highest yield was obtained in the presence of 15 mg of Zn-DABCO@Fe3O4 NPs. After wide screening, the highest yield, best reaction time, and reduction of undesired side products were obtained when the reaction was supported in the presence of 15 mg of the catalyst under solvent-free conditions at room temperature (Table 1, entry 5).
Table 1. Optimizing the reaction conditions for the methylphenyl sulfide

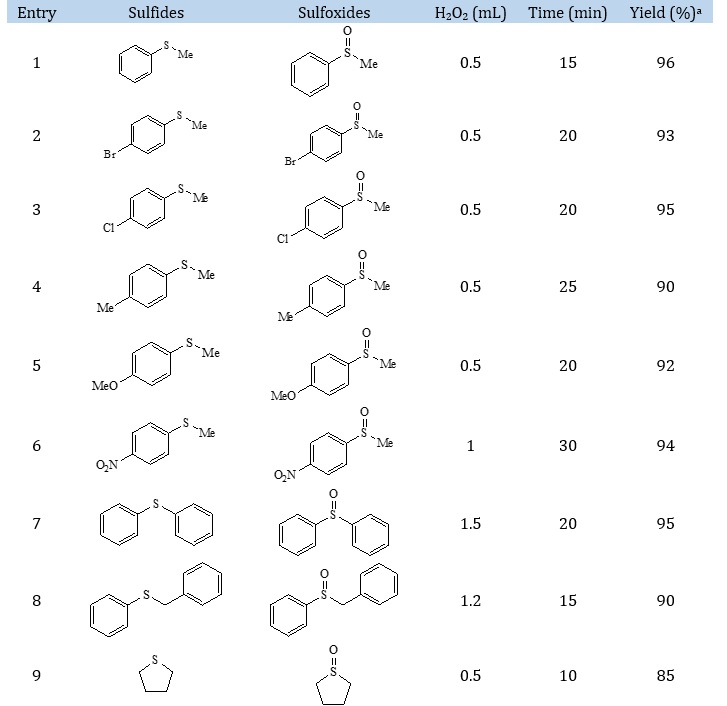
As is evident from Table 2, all electron-donating substituents and electron-withdrawing substituents such as 4-bromo thioanisole (Table 2, entry 2), 4-chloro thioanisole (Table 2, entry 3), 4-methyl thioanisole (Table 2, entry 4), 4-methoxy thioanisole (Table 2, entry 5) were converted into their corresponding sulfoxides in good to excellent yields and short reaction time. Also, 4-nitro thioanisole (Table 2, entry 6) was converted into their corresponding sulfoxides in excellent yield and the slightly longer reaction times. Interestingly, tetrahydrothiophene (Table 2, entry 9), dipropylsulfane (Table 2, entry 10), 4a, 10a-dihydrophenoxathiine (Table 2, entry 15) were converted into their corresponding sulfoxides in short reaction time and good yields. Similarly, diallyl sulfides (Table 2, entries 7, 8 and 12) were oxidized to their corresponding sulfoxides in short reaction time and high yield. All the products listed in Table 2 are known and their structures were determined by 1H NMR, 13C NMR and IR which were in accordance with the literature.
Table 2. Oxidation of sulfides to the sulfoxides in the presence of Zn–DABCO@Fe3O4 MNPs

To establish the catalytic activity of Zn–DABCO@Fe3O4 MNPs, we compared our obtained results for the oxidation of methyl phenyl sulfide with the best of the well-known data from literature (Table 3). As seen in Table 3, oxidation of methyl phenyl sulfide in the presence of Zn–DABCO@Fe3O4 MNPs as catalyst, the higher yields of products were obtained in shorter reaction time and under milder conditions.
Table 3. Comparison of various procedure used for the oxidation of methyl phenyl sulfide with different catalystsa

The recovery and reusability of catalysts are of great concern from economic, environmental and industrial perspectives. In this regard, the reusability of the synthesized Zn–DABCO@Fe3O4 MNPs catalyst was investigated in the oxidation of dimethyl phenyl sulfoxide under the optimum reaction conditions. Since the catalyst can be separated from the reaction mixture using an external magnetic field, it was recovered with a simple magnet after the dilution of the reaction mixture with water. As seen in Figure 5 the catalyst can be reused 8 times in both oxidation reactions with no considerable loss in its initial activity.
 Figure 5. Reusability of Zn–DABCO@Fe3O4 MNPs
Figure 5. Reusability of Zn–DABCO@Fe3O4 MNPs
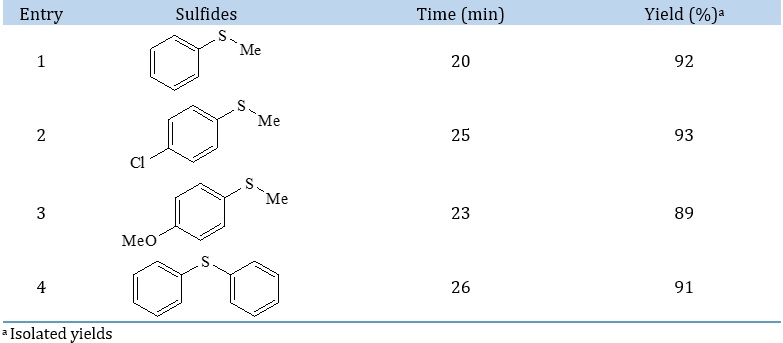
In another study, to recognize the procedure's scalability, we assessed some reactions in 15 mmol of each reactant. The respective results are summarized in Table 4. As demonstrated in Table 4, the reactions were successfully performed at the larger scale without significant loss of the yields.
Table 4. The scalability of the selective oxidation of sulfides to sulfoxides in larger scale (15 mmol of each reactants)
Finally, to demonstrate the high chemoselectivity of the our method, we have also conducted competitive oxidation of various sulfides with O,O-acetals using H2O2 under solvent-free conditions at room temperature (Scheme 3). As shown in Scheme 3, whereas various sulfides were converted to the corresponding sulfoxides, O,O-acetals remained intact under the described reaction conditions.
 Scheme 3. Selective oxidation of sulfides to sulfoxides in the presence of acetals at room temperature
Scheme 3. Selective oxidation of sulfides to sulfoxides in the presence of acetals at room temperature
Conclusion
In this wok, the magnetic heterogeneous Zn–DABCO@Fe3O4 nanocatalyst was synthesized for the first time by DABCO on the magnetic Fe3O4 nanoparticles. The synthesized catalyst was characterized using the XRD, SEM, TEM and VSM analysis. The results revealed that this novel catalyst has high catalytic activity in the selective oxidation of sulfides to sulfoxides under mild reaction conditions. The mild conditions and the use of a green, nontoxic, inexpensive, and reusable catalyst in high yields and in relatively short reaction time are some of the advantages of this method. Also, the involved catalyst can be magnetically separated from the reaction mixture and reused 8 times with no considerable change in its activity.
Acknowledgements
The author is thankful to the Islamic Azad University of Ardabil authorities for supporting this work.
Supporting Information
Additional supporting information related to this article can be found, in the online version, at DOI: 10.48309/JMNC.2023.3.4.