Document Type : Original Article
Authors
1 Department of Chemistry, Payame Noor University, P.O. BOX 3149968143, Karaj, Iran
2 Research Center Karaj Moallem, Karaj, Iran
Abstract
Preparation of Fe3O4 and mica (mica/Fe3O4) nanocomposite as a magnetic heterogeneous nanocomposite is a new and novel idea with limited research. In most of these researches, the combination of salts containing Fe2+ and Fe3+ ions in the presence of mica and an alkaline solution has been used to prepare Fe3O4 and load it on mica. This method may lead to the non-uniform formation of Fe3O4 on the mica. Therefore, it is necessary to find alternative chemical methods that directly use Fe3O4 nanoparticles to bond with mica and form mica/Fe3O4 nanocomposites. The production method in this research is based on the co-precipitation method of superparamagnetic nanoparticles of Fe3O4 (SPMN- Fe3O4) and mica in the presence of SLS as a surfactant and urea as an adhesive. The FESEM image of the nanocomposite showed a uniform and proper distribution of Fe3O4 among the mica sheets. Evaluation of the VSM diagram of nanocomposite confirmed the superparamagnetic properties. XRD analysis of nanocomposite confirmed the existence of two phases, SPMN-Fe3O4 and mica. A simulation of MHT therapeutic operation is performed to explain the MHT process and show one of the applications of mica/Fe3O4 nanocomposite in cancer treatment.
Graphical Abstract
Keywords
Introduction
The use of paramagnetic nanoparticles in timely cancer diagnosis, rapid treatment, and targeted drug delivery to cancerous tissue has been one of the most significant achievements of scientists in the last 20 years. After entering the body, paramagnetic nanoparticles are directed to the cancerous mass [1-4]. Gadolinium paramagnetic nanoparticles have been used for many years as a contrast agent (CA) in magnetic resonance imaging (MRI) and magnetic hyperthermia (MHT) as a treatment method [3, 4-11]. Although gadolinium is an influential factor in creating magnetic properties in body tissues and creating contrast in MRI images, its release has severe toxic effects on the body. For this reason, there is a desire to replace this material with other more biocompatible paramagnetic materials and superparamagnetic nanoparticles (SPMN), such as SPMN-Fe3O4 nanoparticles [5, 12-16].
In the situation where the CA is present in the target organ, applying an external magnetic field around the body causes the alignment of the magnetic vectors of the CA with the external magnetic field. It creates a substantial magnetic property in the tissue. This phenomenon causes the contrast of different tissue parts in MRI images. By removing the external magnetic field, paramagnetic materials lose their alignment [3, 6, 17]. In the MHT treatment method, first magnetic nanoparticles are directed to the target organ. Then by applying the magnetic field and changing it alternately, local heat is created in the target organ. This results in cancer cells being heated up to 40-45 ᵒC and are destroyed [6, 7].
SPMN-Fe3O4 nanoparticles play an influential role in drug delivery to target organs. By attaching specific compounds to SPMN-Fe3O4, the conditions for constructing anti-tumor heterocyclic scaffolds in cancer tissue are provided [4]. However, one of the problems of using nanoparticles is their strong tendency to accumulate and agglomerate, so this problem must be overcome somehow. The presence of magnetic properties in nanoparticles such as SPMN-Fe3O4 increases the tendency to agglomeration. Polymeric shells around nanoparticles can prevent their agglomeration [8, 18]. Another way is to use a nanocomposite of SPMN-Fe3O4, i.e., composing SPMN-Fe3O4 with another material like polymers or geopolymers so that the nanoparticles are placed at specific distances from each other [4]. Using geopolymers to composite nanoparticles is a new idea that has received more attention recently because it has better biocompatibility with the living organism [9, 19-22].
The nanocomposite of mica and Fe3O4 (mica/Fe3O4) has been a nanocomposite since 2000.Liang Xiaojuan's research group prepared this nanocomposite in 2010 by co-precipitating salts containing Fe2+ and Fe3+ to prepare Fe3O4 and mica [10]. Ali Maleki and coworkers have studied Fe3O4 magnetic nanoparticles' size effect on the temperature distribution of tumors in hyperthermia [11]. They prepared mica/ Fe3O4 nanocomposite by co-precipitation as a magnetic heterogeneous material with anticancer characteristics [12]. In addition to chemical methods, physical methods such as laser ablation have also been used to grow Fe3O4 films on mica [13]. However, chemical methods are often more successful due to the possibility of interfering with many variables. So co-precipitation method is still a standard method to prepare mica/Fe3O4 nanocomposite despite the possible disadvantages [14].
Using a suitable stabilizer can help load SPMN-Fe3O4 directly onto the mica without needing to co-precipitate SPMN-Fe3O4 on the mica [15]. The stabilizer can be any biocompatible substance which, in addition to preventing agglomeration of the SPMN-Fe3O4, has a stable chemical connection with them. The use of geopolymers as a biocompatible substance has been recently noticed [16]. Mica is a geopolymeric with a chemical formula of (KF)2(Al2O3)3(SiO2)6(H2O), which is biocompatible without magnetism on which magnetic nanoparticles could be loaded [14].
In the co-precipitation method, two salts containing Fe2+ and Fe3+ ions have prepared Fe2+ and Fe3+ on mica. This method may lead to the non-uniform formation of Fe2+ and Fe3+[23-27]. Therefore, there is a need to find other chemical methods that, instead of salts containing Fe2+ and Fe3+, directly use Fe3O4 nanoparticles to connect with mica and lead to the production of mica/ Fe3O4 nanocomposite with uniform dimensions [12]. So, the magnetic properties and size of SPMN-Fe3O4 prepared in situ, is less controllable. If SPMN-Fe3O4 with specific dimensions and desired magnetic properties is selected from the beginning and then loaded on mica, it can have better results. According to this idea, we present a new, simple,straightforward method to prepare the mica/Fe3O4 nanocomposite in this research.
Experiments
Material and instrumental
SPMNN-Fe3O4 with dimensions of 20-30 nm has been purchased. Mica with dimensions of 2.5 microns, sodium lauryl sulfate (SLS), and urea were prepared with analytical grades. Laboratory equipment used to prepare mica/Fe3O4 nanocomposite includes VGT-1620T ultrasonic bath with 50W power and 40 kHz range and an Alpha model heater Stirrer. The reaction is performed in a flat bottom balloon. The analysis performed on mica/Fe3O4 nanocomposite includes field emission scanning electron microscope (FESEM) with TeScan-Mira III model, vibrating sample magnetometer (VSM) with LBKFB model, and X-ray diffraction (XRD) with PANalytical Company model. Simulation of MHT therapeutic operation is performed to show the importance of using the mica/Fe3O4 nanocomposite in the MRI and MHT process in cancer treatment.
Basic Idea
According to the catalog of purchased SPMN- Fe3O4, a thin shell of polyvinyl pyrrolidine )PVP( with a ratio of 1% w/w has covered the SPMN- Fe3O4. In other words, SPMN-Fe3O4 is encapsulated with PVP (Fe3O4@PVP), Figure 1. This shell partially stabilizes SPMN-Fe3O4, but the percentage of PVP is so low that it does not entirely prevent the agglomeration of Fe3O4. On the other hand, the PVP shell is an organic substance with the formula (C6H9NO)n, and mica is an inorganic macromolecule (geopolymer). The structural difference between PVP and mica creates difficulties in adhesion between them. For this purpose, different materials can be used for a better connection between mica and PVP. According to the investigations, inorganic powders were synthesized via the modified hydrothermal method with the presence of PVP and urea due to the excellent chemical connection between urea and PVP. A mixture of urea and PVP has been used to synthesize and connect between inorganic nanoparticles [17]. Since, in this research, the SPMN- Fe3O4 was directly used to load on mica, it is inevitable to use a surfactant to avoid agglomeration. Sodium lauryl sulfate (SLS) was used to uniformly distribute SPMN-Fe3O4 nanoparticles in solution [18].
 Figure 1. Synthesis plan and surface modification of magnetic nanocomposites with cisplatin
Figure 1. Synthesis plan and surface modification of magnetic nanocomposites with cisplatin
Statistical population
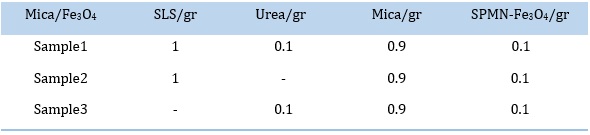
The target sample in this research is the preparation of mica/Fe3O4 nanocomposite with a ratio of 0.1 gr of Fe3O4 to 0.9 gr of mica, along with urea and SLS surfactant. The aim is to achieve a mica/Fe3O4 nanocomposite sample with uniform distribution of Fe3O4 nanoparticles among the mica plates. For comparison, two other samples were prepared, one without the presence of urea and the other without the presence of SLS surfactant, Table 1.
This research was performed as a content analysis to choose the best method for composing mica and Fe3O4 and preparation of mica/Fe3O4 nanocomposite. The method of data analysis is as follows: First, by comparing the appearance of sample 1 with samples 2 and 3, the best sample was selected in terms of uniform distribution, then analytical investigation was applied for the best sample.
Table 1. Mica/Fe3O4 samples
Preparation method of Mica/Fe3O4
The selected method includes loading SPMN-Fe3O4 nanoparticles on mica in an aqueous solvent with the assistance of surfactant SLS and urea. First, 100 mL of deionized water was poured into a 250 mL flat bottom flask containing a magnet and equipped with a thermometer. The flask was heated on a heater stirrer until it reached boiling temperature. Then it was boiled for 5 min to remove the oxygen in the water. Then, 0.9 gr of mica was added to the flask and vigorously stirred for 30 min while the temperature was fixed at 75ᵒC.
After that, 0.1 gr of urea was added to the flask, and the mixture was vigorously stirred for more 30min. Accordingly, 0.1 gr of SPMN-Fe3O4 with 20 mL of oxygen-free boiled water was poured into a beaker and subjected to ultrasonic waves for 15 min in an ultrasonic bath. Then 1gr of SLS was added to the beaker and subjected to ultrasonic waves for 15min to form a uniform suspension of SPMN-Fe3O4 nanoparticles. After that, the suspension of SPMN-Fe3O4 was transferred to a flask containing mica. The flask was heated for 3 hours, and mixture's temperature was kept constant at 75°C and stirred vigorously. Finally, filter paper separated the grayish grayish-black sediment containing mica/Fe3O4 from water. The precipitate was washed several times with ethanol and distilled water. The mica/Fe3O4 was first dried at the environment temperature and then completely dried at 350 °C for 5 h.
The above process was repeated two more times. The second time (sample 2) was in the absence of urea, and the third time (sample 3) was in the absence of SLS. Therefore, the second and third processes investigate the effects of adding urea and SLS, respectively, to the mixture of mica and SPMN-Fe3O4.
Result and Discussion
The best-prepared sample is the sample 1, in which SPMN-Fe3O4 and mica formed a uniform deposit with a homogeneous distribution in such a way that by bringing the magnet close to it, the entire deposit was attracted to the magnet. But in both cases of the sample 2 and sample 3, there was no binding between the SPMN-Fe3O4 and mica, and the SPMN-Fe3O4 was easily separated from the mica by a magnet. So only sample 1 was considered for more analysis.
FESEM of mica/Fe3O4
Figure 2 shows the FESEM of purchased SPMN-Fe3O4, which has a uniform distribution and dimensions below 30 nm. FESEM of sample 1 (mica/Fe3O4) is demonstrated in Figure 3 with different magnifications. Figure 3a and Figure 3b reveal that SPMN-Fe3O4 is well-loaded among mica plates. Figure 3c shows a closer view of this loading.
 Figure 2. FESEM of SPMN-Fe3O4
Figure 2. FESEM of SPMN-Fe3O4
 Figure 3. FESEM of mica/Fe3O4 with different magnification
Figure 3. FESEM of mica/Fe3O4 with different magnification
VSM of Mica/Fe3O4
The magnetic properties of mica/Fe3O4 were measured by the VSM curve at room temperature, Figure 4. The magnetic saturation value (Ms) of mica/Fe3O4 nanocomposite (with 10% Fe3O4) is 10 emug-1. This value, divided by a value of 69 emug-1 for pure SPMN- Fe3O4 nanoparticles [13], is equal to 10/69=0.14. Therefore, the amount of MS in the mica/Fe3O4 nanocomposite with a weight ratio of 10% is logical. Obviously, this number increases with the increase in the ratio of SPMN-Fe3O4 nanoparticles to mica.
XRD of mica/Fe3O4
Accordance to Figure 5, the crystal structure of mica-Fe3O4 nanocomposite was studied using XRD analysis and admitted several phases, including Fe3O4 and mica plates. Considering that the percentage of SPMN-Fe3O4 was only around 10% (0.1g SPMN-Fe3O4 /1g nanocomposite), the intensity of peaks related to SPMN-Fe3O4 is low. However, there are signs of good and uniform distribution of SPMN-Fe3O4 among the mica plates because the presence of SPMN-Fe3O4 is confirmed in the small statistical sample selected for XRD analysis.
 Figure 4. VSM diagram of mica/Fe3O4 nanocomposite
Figure 4. VSM diagram of mica/Fe3O4 nanocomposite
 Figure 5. XRD of mica/Fe3O4 nanocomposite
Figure 5. XRD of mica/Fe3O4 nanocomposite
Analyzing the Urea and PVP role in mica/Fe3O4 preparation
In the report by Lu et al., it is stated that the urea and poly (vinyl pyrrolidone) (PVP) is chosen as the organic compounds to prevent the accumulation of the particles LiNi0.4Co0.2Mn0.4O2 as an inorganic compound [17]. Of course, they did not mention the chemical reaction mechanism between urea and PVP, but it seems that a strong bond between the amine agent in urea and the ketone agent in PVP (Figure 6) causes urea to bind to the PVP shell around the SPMN-Fe3O4. This connection causes SPMN-Fe3O4 to reduce the tendency to accumulate, and secondly, their chemical nature changes from organic to inorganic. The PVP shell around SPMN-Fe3O4 gives them an organic nature. Still, by binding urea to the PVP shell, an intermediate material between organic and inorganic properties, the chemical nature of the shell in SPMN-Fe3O4 becomes inorganic. This change in chemical nature causes SPMN-Fe3O4 to bond better with mica because, in the absence of urea, an excellent chemical bond between iron and mica was not observed.
 Figure 6. Chemical formula of PVP and Urea
Figure 6. Chemical formula of PVP and Urea
Simulation of MHT using mica/Fe3O4 in cancerous liver
The shape of the liver was drawn with a pin in the Corel and transferred to the Photoshop software. Then to show the parameters of temperature, perfusion, nanoparticles, and cancerous mass, the Brush with different models (point and circle) was used, Figure 7. The perfusion variable is shown in red, temperature in yellow, cancer mass in blue, and nanoparticles in black. The therapeutic operation of the cancerous liver is shown in 4 steps in Figure 6. Under the application of the magnetic field, which is done by installing the electrode near the cancerous side of the liver (target), the nanocomposite of mica/Fe3O4 reaches he target through blood perfusion by guiding the applied magnetic force. By applying the magnetic field, the temperature in the cancerous area, which has less oxygen, becomes higher than the other areas. An increasein temperature leads to a gradual decrease in blood perfusion in the cancerous area [1, 18-21]. This process is associated with blood leaving the liver. By repeating this process, the cancerous mass will eventually disappear. This step can be achieved by repeating the MHT operation many times [22].
 Figure 7. MHT destruction of cancerous liver mass in the presence of mica/Fe3O4 nanocomposite
Figure 7. MHT destruction of cancerous liver mass in the presence of mica/Fe3O4 nanocomposite
Conclusion
The co-precipitation method of Fe3O4 and mica in the presence of urea and SLS is a simple, fast, and low-cost method for preparing mica/Fe3O4 nanocomposite. Prepared mica/Fe3O4 nanocomposite in the presence of urea and SLS surfactant was more uniform than the mica/Fe3O4 nanocomposite prepared without urea or SLS. FESEM image of the mica/Fe3O4 nanocomposite showed the uniform and proper distribution of SPMN-Fe3O4 among the mica plates. SPMN-Fe3O4 with uniform distribution and size below 100 nm are loaded among mica plates. Evaluation of the VSM diagram of mica/Fe3O4 nanocomposite admitted superparamagnetic properties with a saturation magnetism Ms = 10 emug-1. XRD analysis of mica/Fe3O4 nanocomposite confirmed the existence of two phases of Fe3O4 and mica. Simulation of MHT therapeutic operation is useful for explanation of the MHT process.
Acknowledgments
We sincerely thank all those who provided us with the laboratory results. We thank the Sharif Laboratory Service Center, the Mahamax Service Center in Tehran, and the student research of Moalem for providing us with laboratory space.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Orcid
Ashraf Heidaripour : 0000-0003-0223-5383


