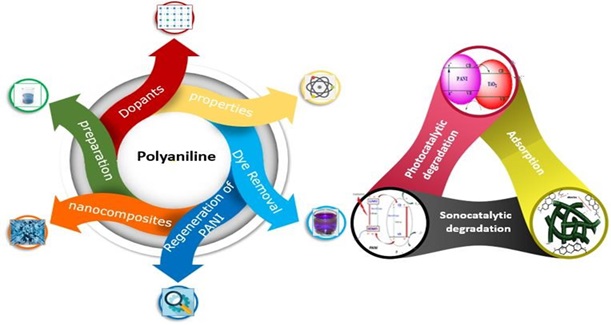
Document Type : Review Article
Authors
Department of Chemistry, University of Wah, Quaid Avenue, Wah Cantt., (47010), Punjab, Pakistan
Abstract
Water contamination by various pollutants, such as conventional pollutants like dyes and other emerging micropollutants, is of great concern. Nowadays, there are a host of processes for the removal of dyes from contaminated water. Due to its greater surface area, mechanical properties, and thermal properties, PANI got wide acceptance. It is prepared by chemical, electrochemical, or photochemical polymerizations. Smart properties of PANI are further enhanced by adding dopants which may be inorganic or organic, like HCl, H2SO4, and nicotinic acid. Due to π conjugated electrons, PANI nanocomposites photo-catalytically degrade dye components from wastewater. However, adsorption is the most efficient and promising one. These composites become good hosts to adsorb dye components on their surface by cationic π-interaction or anionic π- interaction. The unique doping and dedoping properties of PANI change its morphology, pore size, and surface charge, enhancing the adsorbate-adsorbent interactions and the adsorption performance. The adsorption mechanism of dye on the surface of the PANI nanocomposite involves H-bonding, van der Walls interactions, covalent interactions, and electrostatic interactions. In the end, future perspectives on removing dyes using PANI are discussed.
Graphical Abstract
Keywords
Introduction
Water in its pure form is essential for human beings and other living creatures. Water pollution is a global environmental issue, especially for ground waters [1]. Water pollution is caused by adding foreign materials that spoil its quality [2]. These materials include inorganic substances, organic dyes, sewage waste, industrial waste, and sediments [3]. Among these materials, “Dyes” are crucial in polluting water. Dyes are organic-colored compounds having functional groups such as chromophoric groups (NR2, NH2, NHR, COOH, and OH) and auxochromic groups (N2, NO & NO2) [4]. Dyes show different colors due to various structural diversities [5].
Dyes are classified into azoic dyes, reactive dyes, vat dyes, Sulphur dyes, acidic dyes, basic dyes, disperse dyes, and direct dyes [6]. Over 10,000 commercially existing dyes and 1M ton dyes are prepared annually, containing 50% as a textile dye [7]. About 10% of dyestuff is discarded after drying and processing [8]. 2% of the dye component entered the aqueous matter and caused pollution [4].
Long-term usage of water-containing dyes causes severe health issues. Dyes are carcinogenic and mutagenic. These dyes can cause cancer in the kidney, bladder, and liver. They can also cause respiratory issues and skin irritation [9]. Due to dyes' cationic/anionic nature, they reduce the amount of oxygen in the water, which is fatal for humans and aquatic life [10].
Many methods are being used to remove dyes from wastewater, like catalytic degradation, adsorption, flocculation, coagulation, electrochemical treatment, reverse osmosis, ion exchange, and peptization [11-13]. Among all these techniques, adsorption being eco-friendly, biodegradable, and cheap process, is an efficient technique for removing dyes. Adsorbents used for this purpose are activated alumina, natural materials, and metal oxides [14]. However, these adsorbents are less efficient, expensive, and have less surface area for adsorption [15].
Nowadays, polymer-based adsorbents got scientific attention due to their solid binding affinities and vast pore structure [16]. They have good mechanical and chemical properties than all other adsorbents having low cost, obtained from waste materials [17]. These materials contain influential functional groups on their surface, which adsorb different species [18].
Among all polymeric adsorbents, conducting polymers like polyaniline (PANI), and its derivatives polythiophene and polypyrole (PPy) got more attention due to their highly porous structure, high conductivity, regeneration, and non-toxicity [19]. PANI is present in polyaniline base, lucoemeraldine, emeraldine, and pernigraniline [20]. PANI has a surface area of 36.2 m2g-1 [21]. It has poor solubility and a less effective surface because of the large aggregation of chains, which restricts its wide use [12].
Polyaniline is engineered with metal, metal oxides and carbon-based materials to enhance its properties. These nanocomposites may be PANI/PANI nanocomposite, PANI/gold nanocomposite, PANI/GO nanocomposite, PANI/TiO2 nanocomposite [22-23]. Due to their unique properties, PANI-based nanocomposites can be used widely for dye treatment. Abbasian et al. [24] used cellulose/PANI composite to remove direct red the 23, the adsorption capacity was 56 mg-1. Ning Wang et al. [25] used TiO2/PANI nanocomposite and removed acid red G with an adsorption capacity of 445.54 mg-1. Deola Majhi et al. [26] reported 555.5mg-1 adsorption capacity of PANI/sodium alginate for methylene blue removal.
To the best of our knowledge, a limited amount of work has been reported for the adsorption of dyes from water using nanocomposites of PANI. This review paper aims to study and elaborate on the formation of PANI via different methods and the use of PANI modified with natural materials, carbon-based materials, and inorganic materials to remove dyes from water. Regeneration of adsorbent and the dyes' adsorption mechanism on the surface of PANI nanocomposite is also discussed and overviewed.
Structure of Polyaniline (PANI)
In 1862, a London College professor, Henry Latheby reported the first-ever synthesis of PANI via electrolysis of aniline sulphate (AS), which was then treated with a reducing agent. Initially, it was termed aniline black because of its dark color [27]. PANI is a polymer formed by the polymerization of aniline, which is a benzene derivative. PANI exists in three different states, which is why it is most widely used in many areas [28, 29]. In lucoemeraldine, x=0 means it contains only benzenoid form, which is completely reduced and contains only the -NH group. This form is colorless and insulator as well [30]. In emeraldine base, x=0.5 means it has quinonoid and the benzenoid structure is partially oxidized. It has blueish color and insulating properties. In pernigraniline, with an x=1, is an entirely oxidized form containing only the -N- group. It has a purple color and is an insulator in nature. In emeraldine salt x=0.5, it is partially oxidized and neutralized. It contains -NH and N groups. It has green color and is one of the finest conductors of electricity [07]. The proportion of benzenoid and quinonoid in emeraldine salt can be determined from the ratio of the intensities in the FTIR spectrum where the benzenoid peak appears near 1460 cm-1 quinonoid appears near 1560 cm-1 [31].
 Figure 1. Forms of PANI [31]
Figure 1. Forms of PANI [31]
Preparatory Methods of PANI
Polyaniline is prepared by polymerization of aniline via Electrochemical polymerization and Chemical polymerization, Photochemical polymerization, and plasma polymerization. All methods have their advantages and disadvantages.
Chemical method
Chemically, polyaniline is prepared by either in-situ or ex-situ polymerization. In in-situ, nanoparticles of PANI are produced. Toluene is treated with sodium dodecyl sulphate (surfactant) with constant stirring. Sodium dodecyl sulphate (SDS) is dispersed in the toluene solution [32]. Then APS (ammonium persulphate), an oxidizing agent, is added to the reaction mixture, capturing proton from aniline. Aniline's polymerizationhas then proceeded via drop vise addition of HCL or H2SO4. The reaction mixture is left for 24 hours under constant stirring to obtain a polymer. This method is useful for the bulk production of PANI. High molecular weight PANI can also be obtained by keeping the temperature at about 0 °C [33]. Zare et al. [29] reported the free radical mechanism of the chemical preparation of PANI.
 Figure 2. Two simple topologies: (a) hcb, (b) dia [59, 60]
Figure 2. Two simple topologies: (a) hcb, (b) dia [59, 60]
Electrochemical Method
Letheby developed the first electrochemical synthesis of PANI in 1962 [33]. In the electrochemical treatment of PANI, thin polyaniline films of desired shape and size are deposited [29]. Polymerization is usually done in one compartment, with a pt counter electrode, AG/AgCl reference electrode, and graphite as a working electrode. Monomer (aniline) and electrolyte (HCL) are taken in a compartment. If the aniline monomer is taken in small amounts, then the product formed is of lower yield. If monomer is taken in large amounts, yield also fluctuates due to the electrode's limited surface for PANI deposition. Therefore, a limited amount of monomer is taken [34, 35]. The constant potential between 0.5V and 0.9 V is applied versus the reference electrode. Electrode depositions occur at graphite electrodes. The product formed is washed and dried in a vacuum oven. Ehsan Nazarzadeh Zare et al. [29] reported the free radical mechanism of the chemical preparation of PANI.
Plasma Polymerization
This method is useful for producing PANI thin films on the glass surface. Argon is used as plasma gas with a flow rate of 1700 standard cubic centimeters per minute. In this method, the monomer (aniline) is converted into vapor by an Ar tube passing through the monomer vessel. These two gases move through the inlet pipe. Plasma is ignited at around 26Hz. PANI thin films are formed at the glass surface. The polymer produced is uniform, stable, and glow-like; however, it is insulated. Conductive PANI is prepared by adding a dopant like I2, which makes this method more expensive and less commercial [36].
Photopolymerization
Polymerization of aniline to polyaniline can also be done via photopolymerization [37, 38]. Aniline and nitric acid are mixed with a silver nitrate solution. The wavelength of 365 nm is fallen on the sample, which first appeared as transparent, but after polymerization, it appeared dark green. Nitrate was broken, and OH was generated, which led to the PANI formation [28]. Zarrintaj et al. reported the preparation of PANI with mechanism via irradiation path, and properties of PANI [37]. Kuilla et al. reported that oxidative chemical PANI has better properties than electrochemi-cally prepared ones. Electrochemically produc-ed PANI has less crystallinity, higher solubility, lower conductivity, and larger particle size [35].
Effect of Dopants on Properties of PANI
Electrical Properties
Polyaniline can be considered as the finest conductive polymer because it has conjugation where electrons can shuffle and conduct electricity. All other forms of PANI are insulators except emeraldine salt, which is insoluble in common organic solvents. Conductivity of emeraldine salt is 10-2 to 10-0 S/cm [29]. The electrical conductivity of PANI increases with the doping of the EM-Base (insulator form, σ ≤ 10-10 S/cm) and formation of the EM-S (conductive form, σ ≥ 1 S/cm). Conductivity of PANI is explained via “band theory”. After the polymerization of PANI, HOMO (valence band) and LUMO (conduction band) develop. In neutral ] form band gap is 2.3eV, which means low conductivity. With p-doping, the band gap majority charge carriers are holes (positive charges), decreasing the band gap and increasing conductivity. On the other hand, in an n-doping majority of charge carriers are electrons, which also increase conduction [13, 39-42]. M. Federica De Riccardis and Virginia Martina [20] pictorially reported the PANI doping mechanism.
Inorganic acids, such as HCl, H2SO4, HClO4, and H3PO4, and organic acids, such as para-toluene sulfonic acid and dodecyl benzenesulfonic acid, are used for the doping process. While NaOH is used as an undoping agent for EM-salt. Electrical conductivity also depends on temperature, polymeric chain, and doping level. pH also plays an essential role in the electric activity of PANI; Bahar Sökmen et al. [43] reported that the electric conductivity of PANI ceases at pH>4. The electrical conductivity of PANI is calculated by the four-probe method via this equation [15].
σ = ln 2/πd *I/V (1)
 Figure 3. Photoexcitation mechanism
Figure 3. Photoexcitation mechanism
Table 1. Comparison of methods of PANI tuning its morphology

 Figure 4. Mechanism of doping of PANI
Figure 4. Mechanism of doping of PANI
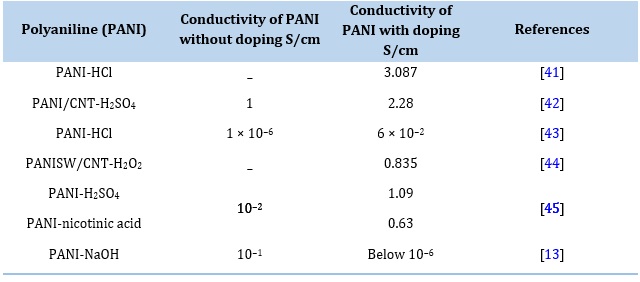
Table 2. The effect of the dopant on the electrical conductivity of PANI
Mechanical Properties
PANI shows good mechanical properties and strength due to its compactness. It has Young’s modulus of 1.91 GPa, strength at a breakpoint of 89.5 MPa, and elongation of 5.88%. Due to its hardness, PANI is used to strengthen natural rubber, carbon nanotubes, and chitosan. For example, a 50% melt blend of PANI with polystyrene provides a tensile strength of 48.73MPa compared to neat polystyrene of 18.39 MPa. Good mechanical stability makes PANI be used as weather resistant or protection against corrosion [13]. The Weightage of PANI present is essential for gaining highly mechanically strengthened material because PANI provides cross-linking in the material and increases its glass transition temperature. Still, after a particular percentage, lumps formation and poor dispersion of the PANI matrix decrease the tensile strength [46-48] reported the effect of the percentage of PANI on the strength of thermoplastic natural rubber.
Table 3. Weight percent of PANI Vs Tensile strength

Thermal Properties
PANI exhibits degradation with an increase in temperature followed by loss of water, dopant, bound water, and primary chain degradation may enhance the thermal properties of PANI. Glass transition temperature (Tg) for uncross-linked PANI is 70 °C and cross-linked PANI is 250 °C. PANI emeraldine base has got excellent heat stability and degrades at nearly 400 oC. In contrast, PANI emeraldine salt has poor thermal stability. The thermal stability of doped PANI depends on the counter anion [13]. Hasan Fisal Alesary et al. reported the temperature stages of weight loss of PANI samples doped with sulphuric acid and nicotinic acid 2-methyl nicotinic acid given in the tabulated form [45].
PANI-H2SO4 has better thermal stability than other PANI. This can be attributed to the strong interaction between PANI and H2SO4. The sulfuric acid molecules form strong hydrogen bonds with the nitrogen atoms of the PANI backbone. These hydrogen bonds stabilize the polymer backbone and prevent it from breaking down at high temperatures. Moreover, the sulfuric acid molecules also act as a cross-linking agent, further stabilizing the polymer chain [49].
PANI is well known for its chemical stability. It is insoluble in most organic solvents. Moreover, it does not involve carbon chain while doping and dedoping. Therefore, it does not react and retains its structure [50]. The properties of PANI are further increased by making its composites with metals, metal oxides, metalloids, non-metals, transition metals, natural materials, and carbon-based materials [51].
Size and Morphology
PANI is found in different morphologies like nanorods, tabular, PANI fibers, and PANI sheets. PANI nanofibers are prepared by homogeneous nucleation, while nanorods are prepared by heterogeneous nucleation [52]. Many organic or inorganic dopants like hydrochloric acid, slfuric acid, perchloric acid, and formic acid influence the size and morphology of PANI materials. The morphology of sulfonate porphyrin-doped PANI is changed from 1D nanotube to 3D cauliflower structure by varying the ratio of dopant [53, 54]. reported that the length and size of PANI hollow-tube morphology is varied by adding different dopants like camphor sulfonic acid. P-toluene sulfonic acid and Tetrakis (4- sulfonate phenyl) porphyrin of 100 nm, 140 nm, and 120 nm, respectively, with less effect on the overall morphology of PANI. SEM and TEM images [53] are shown above.
Table 4. Thermal stablity representation by incorporation of PANI

 Figure 5. PANI-TSA, PANI-CSA and PANI-TSP
Figure 5. PANI-TSA, PANI-CSA and PANI-TSP
PANI Composites
Over the years, the preparation of functionalized PANI nanocomposites attracted great attention. PANI nanocomposites are being used as catalysts, electronic devices, chemical and biological sensors, and adsorbents. Some methods to fabricate PANI nanocomposite are as follows:
Solvent Casting
In this method, PANI is dissolved in a suitable solvent, and a nanofiller is then added in the solution; the solution is stirred to obtain nanocomposite. This method is simple and does not require any apparatus to operate. Polymer weight, polymer structure, amount, stirring, solvent type, and temperature can affect the efficiency of this method [29].
In situ polymerization in the presence of nanofiller
In this method, polymer constituents, i.e., monomer and initiator, are mixed into the nanofiller. This is a better way to enhance the interactions of the PANI composite [54].
In situ nanoparticles formation in the presence of polymer
In this way polymer composite produced has uniform morphology within a polymer matrix. PANI is dissolved in a suitable solvent, and a nanofiller precursor is then added to the solution.
The PANI-based composites can be categorized into two types: binary and ternary [55]. Nanofiller may be natural materials, metals, inorganic materials, and synthetic polymers [56].
Polyaniline/natural materials nanocomposites
Polyaniline can be combined with natural or synthetic fibers, such as coir or glass fiber, to yieldPANI-coated natural fibers via in-situ polymerization. These nanocomposites may be used in gas and biosensing applications [57]. Similarly, the eggshell powder can be blended with PANI to make PANI/eggshell nanocomposite used in ammonia [58]. Agricultural waste like rice husk, sawdust, and juliforia seeds can also be prepared for many applications, especially adsorption. Biopolymers, like chitosan, starch, and alginate, are mixed with PANI to obtain high crystallinity nanocomposites. These nanocomposites are rich in polar functional groups like hydroxyl, ether, carbonyl, and acidic.
Polyaniline/metal nanocomposites
Over the years, PANI nanocomposites with metals got wide attention. Usually, PANI/metal composite is prepared by one-pot synthesis, where PANI acts as a reducing agent for metal ions. PANI/metal NPs nanocomposites, such as Ag, Au, Ni, and Cu, enhance the doping properties. Due to mobile electrons in metals, these nanocomposites also increase the PANI matrix's electrical conductivity in nanocomposite [57].
Polyaniline carbon-based nanocomposites
Carbon-based materials are one of the finest contenders to enhance the properties of PANI. Multifunctional groups in carbon-based materials increase the electrical and mechanical properties of PANI . Polyaniline and carbon-based materials are combined via an in-situ polymerization method to have good qualities. These materials may be activated carbon, graphene nanorods, and graphene nanosheets. Also, these materials increase the adsorption capacity of PANI by inducing multiple functional groups and porosity on its surface [59].
Similarly, PANI ternary nanocomposites are of wide use as that binary composites. These hybrid nanocomposites are due to a variety of functional groups. Increase the electrical conductivity and thermal and mechanical properties of PANI up to a greater extent (Qiangqiang Tan et al. reported PANI/activated carbon/TiO2 nanowires for supercapacitors) [60]. Khan A.A.P et al. prepared lignosulfonate/GO/PANIhybrid nanocomposite as an adsorbent [61].
Dye removal by PANI nps via various techniques
Due to pi conjugated electrons and good thermal stabiare widely used in many areas like electrical and mechanical devices, membrane technologies, supercapacitor electrodes, gas sensors, and biosensors. PANI nanocomposites are also widely used to remove heavy metals and dyes from wastewater. The mechanism of removal of dyes from wastewater is [62].
(a) Adsorption of dyes on the surface of PANI due to its high surface area.
(b) Reduction/Oxidation of dyes components by redox behavior of PANI
Photocatalytic degradation of dye using PANI-based nanocomposites
PANI and PANI composites, due to the lower band gap between the conduction band and valence band, can efficiently donate electrons or transport holes on the irradiation of UV visible radiation. These electrons cause the degradation of dye. Various dye concentrations were taken, and the decreasing absorbance of the dye during the photodegradation experiments was due to its structural breakdown into smaller molecules. PANI provided a large surface area so that more dye molecules could be absorbed on the catalyst surface. In the presence of light, the generated electron-hole pairs distributed the larger dye molecules of high molar mass into smaller molecules of low molar mass [33]. Various factors affecting the capacity of PANI nanocomposite for photo catalytical degradation of dye depend upon the solution's pH, catalyst dose, Dye concentration and temperature [63]. The PANI composite can be recycled and regenerated via centrifugation, which can be used for up to six cycles. Varun A. Chabbra et al. [64] used PANI/PbS Quantum Dots (QD) nanocomposite structure for visible-light-driven photocatalytic degradation of rhodamine 6G up to 87%. Jolly Bhadra et al. 2020 reported the photodegradation mechanism of organic dye removal.
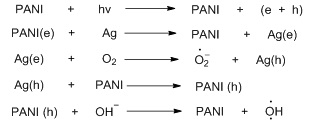
Sono catalytic Degradation of PANI-based nanocomposites
An ultrasonic generator is used to generate ultrasonic waves with a frequency of 40kHz and power 500 W. The dye mixture is sonicated under ultrasonic waves for 30 min. A change in the dye's color indicates the dye's degradation using ultrasonic waves. Degradation efficiency can be calculated via this formula, degradation efficiency (%) = C’-Ct /C’ * 100. Various functional groups are degraded, and degradation efficiency was 446.6 mg-1.
Dyes removal from wastewater using PANI membranes
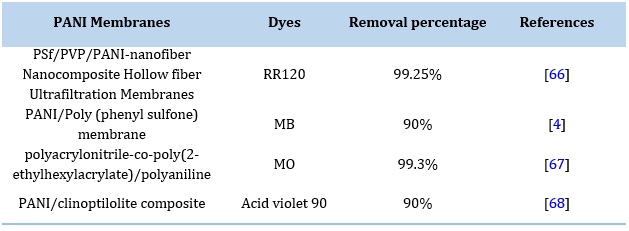
PANI-modified membrane nanocomposites have a more excellent capability of adsorbing dyes components on the surface than un-modified membrane material. These coated membranes have recyclability and stability. PANI hollow surface membranes are preferred over other membranes due to their high surface area, mechanical stability, and easy handling. These membranes or hollow fiber membranes show good photocatalytic activity for removing dyes. Dye adsorption is done by reducing the air gap and varying the content of the PANI nanocomposite. Researchers had used a polysulfate-based PANI/TiO2 nanocom- posite hollow fiber membrane and removed reactive orange 16 with up to 96% efficiency [65].
Table 5. Water decontamination ability of PANI based maerial
Dyes removal from wastewater using PANI by Adsorption
Adsorption is the accumulation of adsorbate (dye) on the surface of the adsorbent. The surface solid or liquid that accumulates gas or liquid molecules is called an adsorbent. Due to its low cost, biodegradable nature, and economical, adsorption is a very acceptable method to remove dyes from wastewater [69]. PANI nanocomposites are one of the best adsorbents for adsorbing dyes due to their high surface area and porous nature. PANI has polar functional groups on its surface to adsorb dye-containing charges on its surface. These polar groups adsorb dye components depending upon the PZC of the composite. pH<PZC, greater the possibility of accumulation of basic dyes on the PANI surface and vice versa. Acidic dyes, basic dyes, direct dyes, disperse dyes, vat dyes, and reactive dyes are significant dyes.
a. Acidic Dyes
Acidic dyes have a negative charge on their surface and are termed anionic dyes [70]. For example, acid blue 25, acid red 57, methyl orange, and Congo red due to negative charge on their surface. These dyes bind with species containing the positive charge. Emeraldine salt is partially oxidized and neutralized and contains -NH and -N- groups on its surface. Also, the pi-conjugated electrons on the surface of the PANI nanocomposite make the dyes land on their surface. J. RAFFIEA BASERI et al. [71] used sawdust-coated PANI nanocomposite to remove acidic violet 49, and the adsorption capacity was 96.84%.
b. Basic dyes
Basic dyes are termed cationic dyes with a positive charge on their surface. For example, asic red 46, malachite green, basic yellow 28, methylene blue, basic brown, and basic red 9. As the PANI emeraldine salt base has NH+ group so it can vibrantly adsorb basic dyes on its surface. The interaction between the PANI and dye can also be ionic, hydrogen bonding covalent, and dipole-dipole. Anwar ul Haq et al. [72] used PANI/Fe3O4 nanocomposite to remove basic blue 3 with maximum removal of 78mg-1 of dye. Research data show that PANI Almond shell bio nanocomposite shows 190.98mg-1 uptake of Orange G [73].
c. Azo dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryls. They are a commercially important family of azo compounds, i.e., compounds containing the linkage C-N=N-C. Due to good degradation properties, PANI nanocomposites first cause the N=N bond, followed by the adsorption of azo dyes on the surface.
Table 6. Comparison Dyes Removal Using PANI Nanocomposites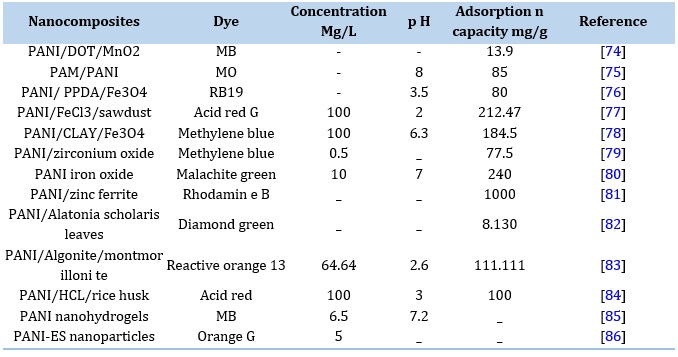
Adsorption Mechanism
The structure and morphology of PANI-based adsorbent and adsorbate predict the adsorption mechanism of the dye adsorption. Mechanisms can be physical adsorption and chemical adsorption. The adsorbents containing chelating groups, such as amine, amide, oxime, hydroxyl, thiol, and carboxylare good candidates for removing pollutants. If the adsorbent surface is negatively charged, it would be efficient for accumulating cationic dyes. i.e., asic red 46, malachite green, basic yellow 28, methylene blue, basic brown, basic red 9; if the adsorbent is positively charged, then it would adsorb anionic dyes, say acid blue 25, acid red 57, methyl orange, and Congo red based on the interaction between adsorbent and adsorbate, adsorption can be monolayer or multilayer. If interactions are just electrostatic, then it would be multilayer adsorption. The benzenoid and quinoid polyaniline rings form π -π interaction with aromatic rings of dye molecules, and electrostatic interaction and hydrogen bonding might also play a role crucial role in the adsorption mechanism of rhodamine B and Congo red. In the case of chemical complexation between adsorbate and adsorbent, there would be [87]. According to Javadian et al. [88], adsorption of anionic dyes onto the polyaniline gamma Al2O3 showed physisorption occurred through the ionic attraction between the cationic amino group of protonated polyaniline gamma Al2O3 and a sulfonated group of anionic dyes, viz., reactive red 194, acid blue 62, and direct blue 199. Abu Nasar et al. [57] showed a multilayer adsorption mechanism of methylene blue on the surface of PANI.
 Figure 6. Adsorption mechanism of MB on PANI
Figure 6. Adsorption mechanism of MB on PANI
Regeneration of Adsorbent
To make the adsorption process more economical, it is necessary to regenerate the spent adsorbent and the dye. They can be regenerated for further processing and applications. PANI, due to its biodegradable nature, can also be recycled. Recycled PANI or PANI nanocomposite also has excellent adsorption capacity for adsorbing dyes on their surface. Regeneration is done by water washing and oscillation treatment. Depending upon the dye and counter ions ionic product, the adsorbent is treated with HCl and NaOH to remove dye from the adsorbent surface. External factors like pressure, and temperature, also play an essential role in the desorption mechanism. Also, potentiometric, and electrochemical treatments are useful for desorbing dyes for adsorbent surfaces [89-91]. Suomi Dutta, Kunal Manna et al. [92] used alkaline NaOH to regenerate PANI/Fe2O3 nanocomposite to remove Arsenic from wastewater. About 98% and 83% of As(III) were removed in the first and third adsorption cycles. Xiao Li Ma et al. [93] reported that the removal efficiency of Cr (III) on the surface of PANI/fiber balls is about 90% for several cycles .
Table 7. Removal efficiency comaparsion of the virgin & regenerated PANI based material

Concluding Remarks and Future Perspectives
This review overviews inexpensive, highly effective nano adsorbents based on conducting polymers. Among different conducting polymers, PANI and its derivatives have been widely used to prepare effective nano adsorbents for removing dyes from wastewater/aqueous solutions. Due to their mechanical and thermal stability and high surface area, PANI nanocomposites make the dye components land on their surface efficiently. Adsorption is considered one of the most effective techniques for water treatment due to the flexibility and simplicity of design. Different types of dopants, say acidic (HCl) or basic (NaOH), tune the morphology, structure, and properties of PANI up to a greater extent and so the adsorption efficiency.
It is anticipated that a variety of dopants can further improve the adsorption properties of PANI nanocomposites. Considering the biodegradable nature of the adsorbent, easy preparation, readily available, and good adsorption process, PANI, in the future, must be used broadly to remove dyes from polluted water.
Acknowledgments
The authors acknowledge Chemistry Department, University of Wah , Quaid Avenue, Wah Cantt., (47010), Punjab, Pakistan for supporting this work.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Orcid
Fawad Ahmad : 0000-0003-2404-5572